

Their general electronic configuration is (n – 2) t 0–14 (n – 1)d 0–2 ns 2. They are also called f-block elements. Lanthanoids (the fourteen elements after Lanthanum) and actinides (the fourteen elements after actinium) are called inner transition elements. They show variable oxidation state and form coloured ions. They have general electronic configuration (n-i) d 1–10 ns 0–2. These are the elements of group 3 to 12 in the centre of the periodic table. They are elements of s-block and p-block. The elements of group! (alkali metals), group 2 (alkaline earth metals) and group 13 to 17 constitute the representative elements. “The properties of elements, as well as the formulae and properties of their compounds are periodic functions of atomic number of the elements”. “The properties of the elements, as well as the formulae and properties of their compounds depend in periodic manner on the atomic weight of the elements”.
(CC BY-NC-SA anonymous by request).He could not classify all the elements discovered at that time. Thumbnail: Ionization energies superimposed on a periodic table. 2.S: Elements, Atoms, and the Periodic Table (Summary) To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms and ask yourself how they relate to the topics in the chapter.2.E: Elements, Atoms, and the Periodic Table (Exercises) These are homework exercises to accompany Chapter 2 of the Ball et al.Some characteristics of the elements are related to their position on the periodic table.

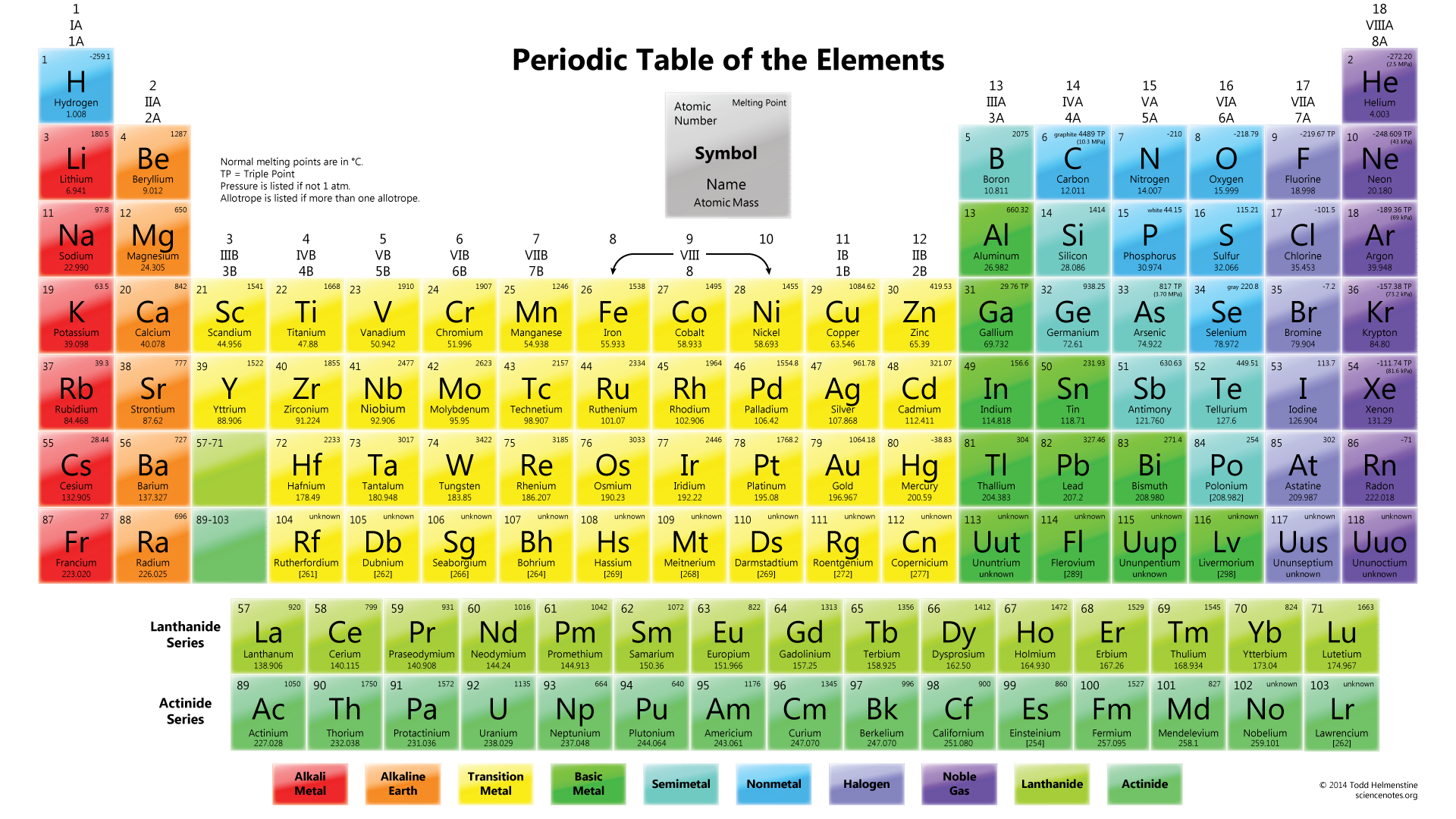
2.7: The Periodic Table The chemical elements are arranged in a chart called the periodic table.2.6: Arrangements of Electrons Electrons are organized into shells and subshells about the nucleus of an atom.2.5: Atomic Masses Atoms have a mass that is based largely on the number of protons and neutrons in their nucleus.Isotopes are atoms of the same element that have different masses. 2.4: Nuclei of Atoms Elements can be identified by their atomic number and mass number.Protons and neutrons are grouped together in the nucleus of an atom, while electrons orbit about the nucleus. 2.3: The Structure of Atoms Atoms are composed of three main subatomic particles: protons, neutrons, and electrons.The modern atomic theory establishes the concepts of atoms and how they compose matter. 2.2: Atomic Theory Atoms are the ultimate building blocks of all matter.Chemical elements are represented by a one- or two-letter symbol. 2.1: The Elements All matter is composed of elements.Unprotected by enamel, a tooth will start to decay, thus developing cavities and other dental problems.

Acids found in some foods or made by bacteria that feed on food residues on our teeth are capable of dissolving enamel. It has to be hard so that our teeth can serve us for a lifetime of biting and chewing however, tough as it is, tooth enamel is susceptible to chemical attack. 2.0: Prelude to Elements, Atoms, and the Periodic Table The hardest material in the human body is tooth enamel.


 0 kommentar(er)
0 kommentar(er)
